| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| 1 | ||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| 23 | 24 | 25 | 26 | 27 | 28 | 29 |
| 30 |
Green tea is increasingly popular in the United States and Europe. A cup of green tea contains three times less caffeine than a cup of coffee and green tea's mild flavor provides little temptation for the addition of calories in the form of cream and sugar. And, like many products in need of a marketing boost, green tea has been the subject of a wide array of claimed health benefits.
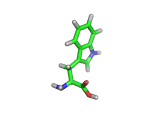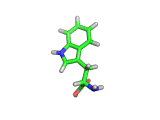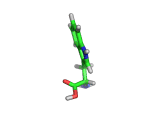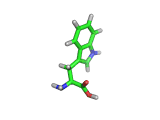
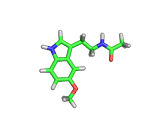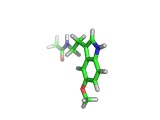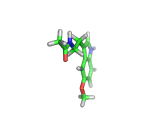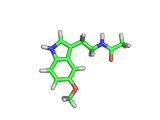
Many of the green tea health claims revolve around polyphenols such a kaempferol which is the shown in image below. Kaempferol is a flavonoid found in many plants. Indeed, interestingly, kaempferol is present in black tea in similar quantities to those seen in green tea, so if kaempferol is indeed responsible for health benefits, these should be observed with black tea as well.
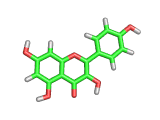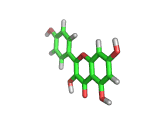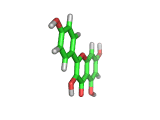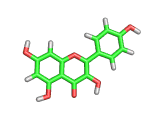
The kaempferol molecule possess delocalized bonding like that seen in benzene and many oxygen centers (shown in red in the image). With these attributes, the molecule is able to absorb electrons from free radicals and prevent the free radicals from causing chain reactions of broken bonds within a cell. This anti-oxidant property may be the origin of the beneficial properties of kaempferol, if indeed these are scientifically confirmed.
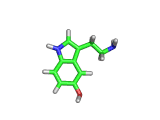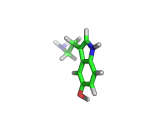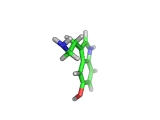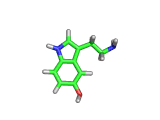
The molecule above is tryptophan, an amino acid famed as a constituent of turkey meat.
In the body, and in the brain in particular, tryptophan is converted to serotonin (shown below) and then to melatonin (shown at the bottom of this article). Melatonin certainly makes you feel sleepy, and it is sometimes taken by long distance travelers to reset their internal clocks when dealing with time differences. In the United States melatonin is sold as a food supplement, while in Europe it is not as freely available.
However, anyone eating carbohydrates is actually dosing up on melatonin.
And although turkey contains plenty of tryptophan so do chicken, beef, and other meats. So tryptophan is commonly present after a meal. It is carbohydrate molecules and the metabolism of carbohydrates which increases the amount of tryptophan in your brain after a hefty holiday feast. This is because carbohydrate breakdown promotes the transport of tryptophan across the blood-brain barrier.
Although the molecule making you sleepy isn't specifically from turkey, it is tryptophan which makes you sleepy after a large meal.
For the first time glycolaldehyde, a very simple sugar, has been found in a star-forming region of the galaxy. That means a modestly complicated organic molecule exists where there are likely to be planets that could support life. This isn't news of an alien life form, it is the detection of a small organic molecule, related to the simplest amino acid glycine, thousands of light years away from the planet earth.
Glycolaldehyde has been seen before but in regions where there are no stars and therefore no planets.
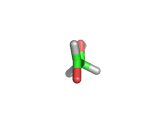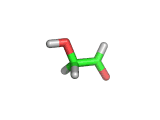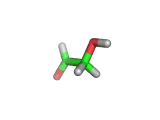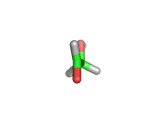
Glycolaldehyde, shown above, was detected by the wavelengths of electromagnetic radiation that it absorbs. Careful analysis of radio telescope data revealed, via the presence of three characteristic lines, that glycolaldegyde was out there.
As you can see from the image, glycolaldehyde is a simple molecule. But despite its simplicity it can be used to construct other more complex molecules, for example, it can react with propenal to form ribose, a constituent of Ribonucleic acid (RNA).
Although a molecule like glycolaldehyde is a building block, this is an important discovery. It means that the diverse organic chemistry that we see on earth is quite possibly present elsewhere in the galaxy. This is something that has long been suspected, on the basis of careful analysis of the contents of meteorites which land on earth, for example. However, glycolaldehyde indicates that there is organic complexity out there amid the stars and where there is complexity there will be interesting discoveries!
Water, water, every where,
Nor any drop to drink.
Or perhaps that should be, all of it fit to drink. The space station astronauts will soon be enjoying refreshing, recycled water. This will enable the space station to support a larger number of occupants with the same amount of water. Additionally, there will be a reduction in the amount of effluent which needs to be handled to keep the space station operational.
While the recycling of water might seem a desperate measure, it is worth remembering that virtually all the water on earth has been 'used' several times. Those few water molecules that haven't been 'used', have probably just been created by the combination of hydrogen and oxygen atoms, and even those atoms have probably been through many cycles themselves.
So water re-cycling is nothing new.
Here are some of the things that may have happened to the last water molecules that you consumed:
A water molecule has two hydrogen atoms and one oxygen atom. Each water molecule can participate in 4 hydrogen bonds, two involving the oxygen atom and one for each of the two hydrogen atoms. (The image above shows hydrogen bonds between water molecules in ice, the hydrogen bonds are shown as light blue cylinders.) This network of hydrogen bonds for each water molecule gives rise to distinct three dimensional structures, such as the structure of ice shown above. The hydrogen bonds make the forces between water molecules strong. Consequently, water is a liquid at room temperature, while similarly sized molecules (like methane for example) are gases.
On the surface of water, the strong forces hold molecules rigidly in place, giving rise to surface tension. In turn, the surface tension of water gives rise to the liquid properties that we rely on, from the size and shape of water droplets, to our ability to improve water's wetting ability with detergents.
Occasionally, the marvels of simple water molecules and the structure of liquid water inspire people to become carried away with molecular flights of fancy. So, for example, enterprising individuals sell bottles of 'special' water, in which, it is claimed, unique clusters of water molecules are present. However, although you would not know it by the sales of such products, it is impossible to have water molecules do your bidding in a bottle. Water is chaotic water. Even the expensive bottle of designer water, with the 'specially produced water clusters for extra hydration' is just a bottle of recycled molecules that has been filtered through several organisms and possibly your neighbors on its way to its fancy bottle!
I was impressed to read that a significant improvement in the ability of computational methods to compute the structure of crystals has been made. The innovation comes from Marcus Neumann and Marc Perrin who show how first principles density functional theory (DFT) calculations can be combined with empirical attraction potentials to rank hypothetical crystal models accurately on the basis of calculated energies.
For molecular crystals this methodology has significantly improved the predictions which are obtained from computational methods.
For inorganic systems, where dispersion forces are less important, it has long been possible to throw the constituent atoms into a suitable box, and use energy minimization to obtain a prediction of the energy of the system. The image below provides and example of such a prediction for the rather simple case of TiO2 in its rutile form. The text in white beneath each structure shows the energy of the system, which declines in successive models, to the final energy, around 223eV, in the final structure, which is shown bottom right.
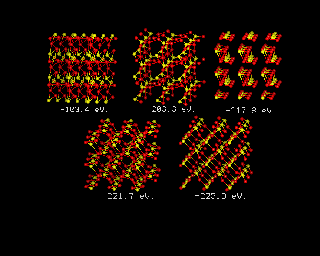
For both molecular crystals and inorganic systems, the theoretical methods can be improved still further, for example: It is possible to include temperature effects, either directly through the use of molecular dynamics, or using a harmonic model. The accuracy of binding energies can be improved. The speed of calculations can be increased.
However, as Neumann and Perrin, and their coworkers, Kendrick and Leusen have shown, computational techniques are now capable of revealing the details of molecular crystal packing in advance of experiment. The results of the computations will not be borne out on every occasion. However, the technology is advancing, and will continue to advance.
The molecule below is enkephalin. It is a peptide, that is, it is composed of amino acids, connected together by peptide bonds. Protein molecules are formed in precisely the same way. Peptides are smaller molecules than proteins and are often used in signalling within our bodies.
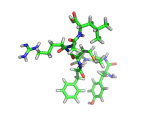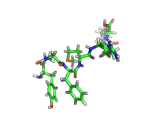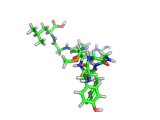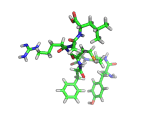
Enkephalin is the molecule which is believed to make you feel good when you have exercised or reached an objective. It interacts with the same receptors as morphine and heroin. The next time you are on an emotional high, having just scored a basket, point, goal, or touchdown, enkephalin is the molecule responsible for your sense of well being.
If you compare the enkephalin structure with the structure of heroin you will see that there are similarities. Both molecules possess aromatic ring structures, hydroxyl groups and positively charged regions. Similar structural feature lead to similar behaviors. Of course, the natural enkephalin molecule leads to a short lived natural euphoria, which our minds can cope with. Heroin on the other hand, which is a more robust and less flexible molecule, leads to intense feelings of pleasure and terrible addiction.
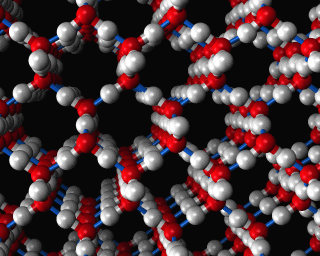
Zeolites, like ZSM-11 which is shown below, are important industrially. Zeolites possess pores in their structures which have sizes similar to the size of molecules. Zeolites can also be made to have acidic properties. So, when an acid catalyst is required which can target certain sizes and shapes of reactant and product molecules, zeolites find a range of applications.
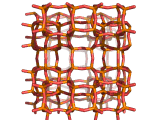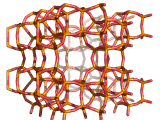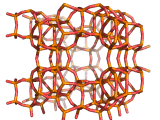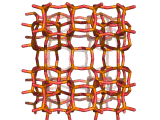
In fact, all of the molecules of gasoline in a typical car have encountered a zeolite catalyst like the one shown above. Oil refineries use zeolite acid catalysts to modify, or crack, crude oil and maximize the output of molecules suitable for use as fuels.
Zeolites are also used in creating specific isomers, avoiding the need for separations following acid catalyzed reaction.
If you look at the image of ZSM-11 you will see why it is special. It contains openings which can admit molecules of certain sizes. If a molecule is too large it will not be able to gain access to the interior of the zeolite, and as the acid sites which the zeolite possesses are in its interior, no acid catalyzed reactions will occur. However, if a molecule is small enough to enter a zeolite it will be rapidly attacked by the protons of the zeolite's acid sites, and interesting chemistry will occur.