| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| 28 | 29 | 30 | 31 |
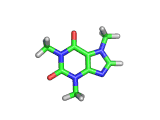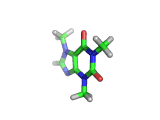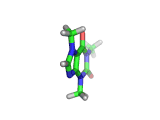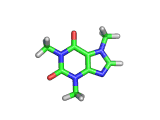
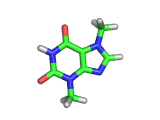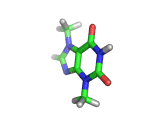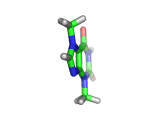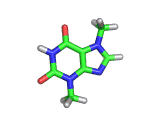
The molecule above is caffeine and the molecule below is theobromine. These molecules give the bitter taste to coffee and tea, in the case of caffeine, and chocoloate, in the case of theobromine. The difference between the two molecules is small. They differ by a single methyl (-CH3) group attached to their six membered rings.
In addition to giving coffee, tea, and chocolate their bitter, and some might say acquired tastes, caffeine and theobromine act as stimulants. Both molecules are similar in shape to part of a molecule known as cyclic adenosine monophosphate, and this confuses the enzyme whose job it is to convert cyclic adenosine monophosphate to a less active form.
The resulting excess of the active form of cyclic adenosine monophosphate leaves the organism with additional stimulation, often manifest in the form of nervous energy.
The physiological result, or buzz, can be quite addictive; a fact not lost on the purveyors of carbonated sugar waters. Hence soft drink companies routinely mix a little caffeine into their products in order to stimulate their customer base and increase their loyalty.
The theobromine in chocolate is probably the chemical origin of chocolate cravings. When such pangs strike, the nervous system is actually craving the stimulation that surplus cyclic adenosine monophosphate provides, and chocolate provides a convenient and familiar route to achieving this surplus.
Cholesterol, the molecule above, is produced in large quantities (around a gram per day) by your body. In fact, cholesterol is essential for life. Cholesterol is the starting point of the formation of hormones such as testosterone and estrogen. Additionally, cholesterol is an important component of cell membranes and is found in the myelin insulation of nerve cells where it assists in the conduction of nerve impulses by providing an electrical barrier.
As the image above shows, cholesterol has a single hydroxyl (-OH) group and this hydrophilic group is not enough to render the molecule soluble in water or blood. Consequently, in the body, cholesterol is transported in the form of lipoprotein complexes, protein coated particles with insoluble molecules at their core. These lipoprotein complexes are characterized by their density, and empirically an association between low density lipoprotein (LDL) complexes and atherosclerosis, has led to the term 'bad cholesterol' being applied to an increase in LDL relative to HDL in the blood (where HDL is high density lipoprotein, of course).
So, as is often the case, the detail is more interesting than the simplification. Cholesterol is vital for the functioning of your nerves, endocrine system, and cell membranes, and 'bad cholesterol' is actually a measure of the relative concentrations of the different cholesterol transporting agents within your blood stream.
If you were to entirely eliminate cholesterol from your system undesirable and quite probably fatal consequences would ensue.
However, if you have too a high a ratio of LDL to HDL, your cholesterol is showing up in the form associated with heart disease and you would be well advised to pay attention and seek to adjust the ratio. Two well known means to effect such a change are exercise and statin drugs. Neither eliminates cholesterol. However, they do change the ratio for the better.
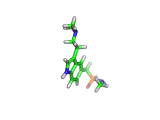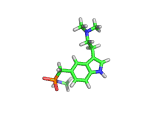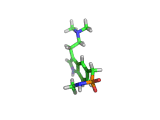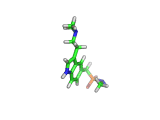
Sumatriptan, the molecule above, is a remarkably selective medicine. It binds to a certain receptor, on the smooth muscles which dilate to cause pressure on surrounding nerves, and rapidly reverses migraine headaches.
Other molecules, such as ergotamine, (which is related to LSD), are also capable of stopping the painful blood vessel dilation that causes migraines. However, these drugs affect blood vessels throughout the body. Such indiscriminate action can interfere with and even damage parts of the body that are functioning normally. Sumatriptan, with its targeted action, is a safer remedy.
Sumatriptan achieves this targeted action by binding to receptors in cranial blood vessels which are intended to interact with serotonin (the molecule shown below). You will see the similarity between serotonin and sumatriptan - they share a common core which is recognized by the receptor. Sumatriptan has been chemically tuned to bind strongly to the specific receptor found in cranial smooth muscle tissues and mimic the effect of serotonin.
