Fri Sep 19 21:51:48 PDT 2008
Fluorite, Uranium Dioxide, and Zirconium Dioxide
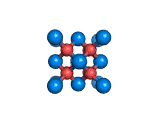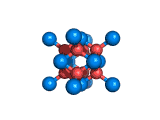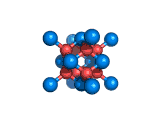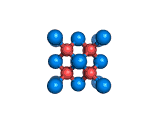
The structure shown above is a small fragment of the fluorite crystal structure. This is a crystal of calcium and fluorine that repeats through space based on the building block which is shown above. The formula of fluorite is CaF2; there are twice as many fluorine ions as calcium ions in the structure. The fluorine ions occupy tetrahedral holes in the lattice of calcium ions. In the image above, the fluorine anions are shown in red. (There seems to be an ancient tradition of displaying negatively charged portions of materials and molecules in red) and the calcium ions are shown in blue (again there is a tradition of coloring positively charged portions of chemical structures in blue).
Fluorite, which is also known as fluorspar, is found in crystalline form in nature and is the feedstock for the product of hydrogen fluoride which is an important industrial chemical. Fluorite crystals are often octahedral in shape.
Two other materials which can occupy the same structure as fluorite are uranium dioxide, UO2, and zirconium dioxide, ZrO2. Uranium dioxide is used in the nuclear industry and zirconium dioxide is used in oxygen sensors. In both materials, vacancies on the anion lattice (the red spheres in the image) allow a certain degree of mobility for ions within their crystal structures. This anion mobility gives rise to some of the interesting properties of fluorite structured materials.