Wed Sep 10 21:10:55 PDT 2008
Defects Add Character
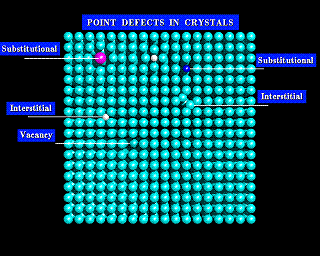
The images below show two models of a simple solid. The first model is not labeled, and at a first glance looks regular. However, if you look closely you will see a number of imperfections in its structure. These defects are labeled in the second image.
Why the interest in imperfections? (You might well ask!). Just as for people, imperfections make solids interesting. Defects give solids many of their most important properties. If you are reading these words on a computer, you are reaping the benefits of defects in silicon. Defects make silicon a controllable semiconductor that can be used to make transistors and integrated circuits.
The simplest types of defects are vacancies; atoms that are simply missing from their normal sites. There are also substitutional defects, where a foreign atom occupies the site of a given atom within the lattice. Finally, there are interstitial defects. Interstitial defects are atoms between the normal lattice sites of the crystal. You will see each of these defects in the diagrams below.
Defects control many of the properties of alloys, semiconductors, and all manner of materials. Imperfections are also implicated in the corrosion of metals, catalysis on surfaces, and the growth of crystals. So, defects are important!
You can find more information on defects in The Molecular Universe site.