The Molecular Universe
Models In Science
|
We have always needed models to understand the complex world in which we live. Models allow our minds to safely explore reality; and models scale tiny and huge objects to a size that we can comprehend and become familiar with. Models are therefore essential in the scientific quest for a rational understanding of the physical universe. Visualization is, moreover, an immense aid to the scientific imagination, as it seeks new relationships and connections between concepts and phenomena - the process which is at the heart of scientific discovery. Read more...
Atoms and Bonding
|
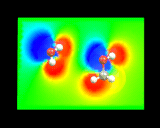
What are the ultimate constituents of matter? This question has always fired the scientific imagination; and it remains the intellectual driving force behind one of the largest scale contemporary scientific activities, high energy physics. The Greeks speculated on the nature of the indivisible atoms of matter; to Newton, atoms were 'massy, hard and impenetrable'. Modern atomic theory was founded by the genius of a Manchester schoolmaster, John Dalton, who suggested that the sparse quantitative chemical data available in the early nineteenth century (mainly concerning the volumes and weights of reacting gases and solids) could be rationalized by postulating that there were different types of atoms corresponding to the different chemical elements; the different atoms had different masses and reacted in simple proportions to give compounds. As the nineteenth century progressed, the concept of the molecule, or aggregate of atoms, took root and, with improvements in the techniques of chemical analysis, the atomic components of key molecules were identified. The idea of molecular shape began to emerge. Thus a turning point in the science of organic chemistry was the development by Loschmidt and Kekule of the hexagonal ring structure for the molecule benzene; Loschmidt's original models. Read more...
- Atoms or What the World is Made of
- Complicated Atoms
- Sticky Atoms
- Molecular Shape
- Reactions Between Molecules
Building Up Molecules
|
Some atomic assemblies are simple. As we have seen, hydrogen atoms stick to each other to form the (H2) molecule. Hydrogen atoms stick to oxygen atoms to form the water (H2O) molecule, to nitrogen to form ammonia (NH3) and to carbon to form methane (CH4) - as shown on the left. Oxygen binds to carbon to form carbon monoxide (CO) and carbon dioxide (CO2), also shown on the left). These and many other compounds based on small molecules are immensely important; for example, water makes up 80% of our body weight and methane is a widely used fuel (being the main constituent of natural gas). CO2 has been implicated in global warming - this small molecule, produced in vast quantities, could change the climate of the world. Read more...
Building In 3 Dimensions

|
Order, regularity and symmetry attract admiration in art, architecture and in the natural world. Of course, disorder and chaos characterise many types of physical entities; and the second law of thermodynamics formulates in precise and quantitative terms the evolution of all systems towards states of increasing disorder. But many natural objects are ordered and symmetric. Crystals have long been an object of fascination to the layman and to the scientist (especially so when rarity adds value, as in gemstones). Typical real crystals are characterised by regular shapes, smooth faces and sharp, well defined angles between the faces. We are also often interested and intrigued by the optical properties of crystals - their colour (or, as in diamond, their high refractive index which causes light to be reflected internally and results in the special glitter and brilliance of the stone); and mechanical behaviour, like the exceptional hardness of diamond, are another stimulant to our curiosity. The starting point of our enquiry in this section is, however, their special geometrical properties of regularity and symmetry. In particular, we will enquire whether the regularity of the external appearance of crystals is reflected in their atomic architectures. Read more...
- Building in 3 Dimensions - Crystals
- More Complicated Structures
- Linking Shapes Together
- Achieving Complexity
- Inserting Atoms Between Octahedra
- Inserting Atoms Between Tetrahedra
- Microporous Solids
- What the Earth is Made Of
The Molecules of Life
|
There is a gulf between inanimate matter and living things. A piece of granite is very different to a plant. A granite rock fragment was formed three billion years ago. Today its modification requires concerted effort and significant expenditure of energy. A plant, composed of carbon dioxide, water and sunlight, can trace its history to a seed or cutting planted in rather recent times. In a year a potted plant on the window ledge will have changed dramatically. Its leaves will have exchanged large volumes of gas with the atmosphere, and if the plant has been cared for, it will have absorbed several gallons of water. The granite fragment will be exactly as it was just after it was formed. Yet the same forces which keep the rock constant permit the living things to change and evolve. Here we look at the molecular basis of the molecules of life. Read more...
Docking and Blocking
|
The important ideas in science need time to gain acceptance. For example, since the late nineteen fifties that the precise images of crystallography have revealed details of the interactions between macromolecules and their molecular targets. It is easy, then, to conclude that the recognition of the importance of such interactions is a recent idea. Yet, in 1894, twenty years before the structure of sodium chloride was solved and more than half a century before any protein structures were known, Emil Fischer, a Dutch organic chemist, was able to propose that enzyme and substrate fit together 'like a lock and key'. Fischer's bold proposal was made after careful study of the effects of enzymes on sugars and explained the precise specificity of enzymes able to distinguish between even the mirror images of the same molecular structure. Read more...
Boundaries and Barriers

|
The behavior of molecules and materials is determined in many instances not by what happens in their interior, but by what happens on their surfaces. Chemical reactions often take place at surfaces - and surfaces can promote chemical reactions, as in the field of heterogeneous catalysis which contributes so much to the chemical industry and hence to our lives. Friction, the overcoming of which consumes huge amounts of energy, takes place between surfaces sliding over each other. The growth of crystals takes place at surfaces; and if we want to prevent it, we need to modify the surface. Computers are guiding and illuminating our knowledge of surface structure and behavior. Read more...
- Surfaces and Interfaces
- Aggregates of Atoms
- Liquids and Glasses
- Crystal Shapes
- Molecules on Surfaces and Catalysis
- Molecules and the Materials Below
- Membranes and Films
- Solutions
Computers, Molecules, and Materials
|
In the 1950s, the distinguished theoretical chemist, Professor Charles Coulson, attempted, together with a colleague, Dr. Bill Moffatt, to undertake a calculation on the nitrogen molecule - a simple species, comprising two light atoms which we know are very strongly bound. Coulson was studying the most fundamental problem in chemistry, the question of why atoms form molecules. The calculation he performed aimed to investigate the bonding characteristics of the molecule - the distribution of electrons around the nuclei and the energy levels of the electrons in the field of the nuclei. It used developments of the quantum mechanical theory describing the distribution of the electrons in atoms that had been formulated by Schrödinger in the 1920s. The technology available to Coulson was a mechanical hand calculator. After several months work, the study was abandoned as it was estimated that it would take another 20 years to complete, which was sufficient to daunt even such a dedicated scientist as Coulson. Read more...