The Molecules of Life
 |
There is a gulf between inanimate matter and living things. A piece of granite is very different to a plant. A granite rock fragment was formed three billion years ago. Today its modification requires concerted effort and significant expenditure of energy. A plant, composed of carbon dioxide, water and sunlight, can trace its history to a seed or cutting planted in rather recent times. In a year a potted plant on the window ledge will have changed dramatically. Its leaves will have exchanged large volumes of gas with the atmosphere, and if the plant has been cared for, it will have absorbed several gallons of water. The granite fragment will be exactly as it was just after it was formed. Yet the same forces which keep the rock constant permit the living things to change and evolve. Here we look at the molecular basis of the molecules of life. In the eighteenth century amid the earliest glimmerings of the dawn of chemistry, natural organic substances, such as the extracts of plants, bone, skin, wood and so on, the substances produced by life, were thought to be fundamentally different from inorganic materials such as rocks, minerals, metals and air. The difference between the constant minerals and the changeable organic compounds, it was supposed, lay in the possession by the latter of a unique vital force endowing them with unique, almost magical, properties.
One of the essential roles of science is demystification, replacing, where possible, vague and unverifiable concepts, like vital force by precise, rational and demonstrable explanations or theories. One of the extraordinary contributions of the chemical sciences to mankind's knowledge has been the demystification of the behavior and nature of living matter. As we shall see, living matter can be understood in terms of the same chemical principles as inorganic matter. Living matter is composed of atoms, in fact a rather restricted range of atoms, and these atoms are bound together to form molecules. Life is undoubtedly complex and diverse, but its molecules, its matter, do not require any new or different fundamental theories. There is no need for a vital force to understand the molecules of life.
However, the thinkers who proposed the vital force were by no means fools. They were responding to the fact that living matter is extraordinary and manifests properties quite different from inorganic materials. Explanation of these facts using the intellectual tools of the time demanded a degree of inventiveness. The rapidly expanding knowledge of underlying chemical phenomena of the time, and the catalogs information available on the molecules of life meant that ingenuity was required to observation.
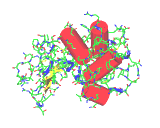
That the molecules of life are special is emphasized by new structural discovery revealing their modes of action. We shall take one initial illustrative example. The unique architectural the lysozyme molecule (shown to the left). This complex molecule (shown in simplified form below) is found in human tears. Its unique shape endows it with unique molecular properties. The complexity of the molecule makes it special. In fact it is a solution to a complex operational problem.
Fixed eyes are effective in simple organisms, but impose a significant impediment to survival for animals that must contend with the challenge of predators or agile prey. The development of eyes able to move within an eye socket marked a significant improvement and demanded, in turn, a lubricant able to bathe delicate optical equipment and ease the friction of motion. The lubricant itself needed special properties, low viscosity so as not to entrap debris, low production cost so as not to consume too large a portion of the organisms food intake, and low opacity so as not to interfere with systems original visual purpose. High on the list of requirements too was the necessity that the lubricant should not harbor bacteria. Tears therefore contain a specific agent that attacks just bacteria, destroying their cell walls. The agent which nature developed for this purpose is lysozyme. It does not effect the delicate membranes of the eye or the eye socket. Instead, it is able to recognize at the molecular level a component of a bacterium's cell wall and, having keyed onto this element, break down the wall destroying the foreign organism.
problem and it is just one of the 10,000 different proteins which make up about half the dry weight of a typical mammalian cell. These special molecular solutions in the form of molecules are among the molecules of life. Similar types of molecule tackle problems in the transportation of gases, the structure of cells and muscles and the regulation of blood flow.
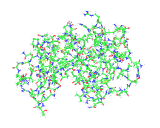
The image on the left shows the structure of lysozyme molecule again, this time all of the atoms of the molecular are shown, include those of hydrogen atoms both in the molecule and in a number of water molecules (in whose presence the molecule operates). The structure reveals the great complexity of the molecular problem solver which nature developed to solve a difficult problem. The complexity is a consequence, a product and indeed a marvelous achievement of millions of years of evolution but it can also be understood in terms of the basic principles of chemistry. The molecules of life achieve must efficiently tackle a vast array of difficult activities within a living organism. As we see in the case of lysozyme and the growing number of structures that are being made available by modern structural discoveries, the molecules of life realize their effects through their complex and unique molecular architectures. How do the molecules of life achieve this complexity?
 |
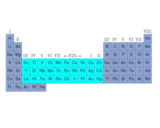
Understanding the extraordinary diversity of function that even the simplest living matter must exhibit provided the root for the vital force theory. The number of elements within living molecules is small. The image on the left shows the periodic table and highlights those element types that form 99% of living things. The vitalists therefore reasoned that complexity must stem from an extra non-elemental ingredient. We now know that nature achieves complexity with the same efficiency that allows common basic building blocks such as transistors and resistors to be linked together to produce devices as varied as computers, toasters and car ignitions from common components. The molecules of life are often chains of atoms. Once you start building with chains, there are so many permutations or possible orderings, that complexity naturally emerges. The image on the left shows some of the simpler molecules that a living organism contains, for example in cell walls and within fatty energy reserves. Lysozyme belongs to one of the most important classes of molecule, the proteins. These molecules are built from a limited number of types of component, the amino acids shown below.
 |
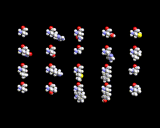 |
Amino acids are linked together in the manner shown below. A water molecule is eliminated between the two aligned molecules, forming a bond known as a peptide bridge. (A similar reaction is responsible for the formation of the chains of molecules which make up nylon). The reaction between two peptides can be reversed making it possible to assemble or disassemble the chain. This makes this class of molecule of life highly efficient. They can be assembled to perform a specific function and then consumed in the production of an entirely different molecular solution. However, to achieve important actions, such as the efficient destruction of aberrant bacteria in lysozyme's case, the correct chain must not only be assembled but the molecule most coil and wrap around itself to produce a finished product. In fact the many of the molecules of life are firstly obliged to interact with themselves, to form the tightly packed molecular machines which perform their assigned functions before they can begin operation. The image above in red shows the extended length of the lysozyme chain in comparison to the dimensions of the coiled active molecule. It turns out that proteins are able to perform the requisite coiling and folding for themselves, without molecular assistance. What is it that makes this happen?
 |
 |
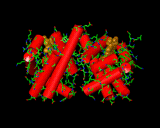
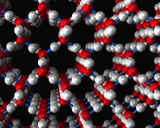
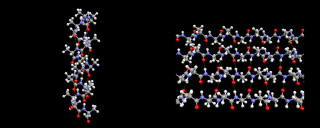
When first formed the protein is in contact with water molecules, indeed almost 80 percent of the typical organism is water, so this is the case for many of the molecules of life. Different parts of the protein structure are then interacting with water from the moment that it is formed. Different amino acids interact with water to varying degrees owing to their differing molecular structures. One of the most important processes is a special type of bonding known as hydrogen bonding. Similar bonds occur between hydrogen atoms and oxygen atoms in water molecules in ice crystals (on the left, for example) and, just as in ice, produce a strong stabilizing force anchoring parts of the protein structure. One example of such bonding is provided by an image below, which shows the alpha helix an important element of protein structural chemistry which was predicted on theoretical grounds before it was seen in nature by Linus Pauling. The patterns which real proteins exhibit can become quite beautiful and complex. Also shown below, is the haemoglobin structure, the protein that is responsible for transporting oxygen through your body from your lungs to where it is required in your muscles and cells. Complex proteins contain an array of different patterns which are dovetailed together to give an overall shape which controls function. Often several separate protein strands are entwined to produce a super complex to achieve a given objective.
 |
 |