What the Earth is Made Of
 |
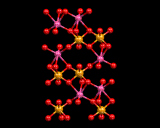
The earth condensed from a dust cloud some five thousand million years ago. The dust contained a wide range of chemical elements, being especially rich in iron, nickel, silicon, magnesium and oxygen. As a consequence, the earth consists predominantly of silicate systems with a metallic core. (The gravitational forces acting on the heavier metallic elements Fe and Ni lead to their concentration in the centre of the earth.) A schematic illustration of the structure of the planet is shown on the left. It comprises three main components: the core, the mantle and the crust. The former is, as noted, metallic, consisting of an alloy (a crystalline substance containing two types of metal, in this case Ni and Fe). The mantle can be subdivided into upper and lower sections. The lower region consists mainly of the mineral magnesium silicate(MgSiO3), but at the immense pressures present at this depth an unusual structure is adopted, in which silicon is six-fold coordinated and the material adopts a structure similar to that of perovskite as shown. This high density structure under normal pressures is unstable and will transform to a structure in which the silicon is tetrahedrally coordinated. At the lower (but still very large) pressures in the upper mantle, tetrahedrally coordinated silicate structures of composition Mg2SiO4 dominate. This interesting compound shows different structures at different pressures, with the spinel structure (in which Si2O7 units are present) adopted at high pressure (greater depths) and the olivine structure at the lower pressures in the outer portion of the mantle. All these mantle minerals contain high concentrations of iron (the ratio of magnesium to iron is typically 10 to 1); the iron is easily accommodated in the structure simply by replacing magnesium atoms.
 |
The thin crust of the earth on which we live is a rich and complex assembly of minerals, again dominated by silicate chemistry. The range of geological processes from vulcanism to erosion have provided a vast and continuing experiment in solid state chemistry that scientists are only beginning to understand. Similarly, understanding of the physical properties of the mantle minerals is yielding an understanding of how the planet works as a whole.
 |
So inorganic chemistry and crystallography have given us a detailed understanding, again of the arrangement of atoms, in the minerals from which our planet is made; in just the same way, organic and bio-chemistry have allowed us to understand at the atomic level the materials of which our bodies and all living things are constructed.
DEFECTS
The perfect crystalline solid represents an extreme of complete order. Such a state is unattainable except in the hypothetical absolute zero. The drive in nature towards disorder - towards higher entropy - means that even if the energy cost is high, elements of disorder must always be present in ordered crystals. Of course at sufficiently high temperatures, the order is lost entirely and the crystal melts. But crystals below their melting point always contain some disordered structures known as defects. And these species are not a mere curiosity. They can exert a controlling influence on many of the most important properties of the crystal, for example its mechanical strength and the rates at which atoms can diffuse through the crystal.
The simplest type of defects are those in which atoms are simply missing from normal sites or additional ones inserted. Both provide effective ways of promoting atomic migration properties. The empty sites (vacancies) allow neighbouring atoms to jump into them, while the extra 'interstitial' atoms can therefore move through the crystal.
A major source of defects in almost all crystals are impurities. Even with the most rigorously careful methods for preparing pure crystals, impurities are inevitably present. They commonly occupy the regular sites of the crystal, and they can have dramatic and profound effects on the properties of the crystal. The classic example is the semiconductor material silicon: when small concentrations of phosphorus impurities are introduced, the phosphorous atoms replace the silicon and form four covalent bonds to neighbouring silicon atoms. But phosphorus has one more electron compared with silicon and the extra electron is easily removed (ionised) from the phosphorus; it may migrate through the crystal and will therefore enhance the electrical conductivity. Silicon is a semiconductor and when phosphorus impurities are included (or doped) into the material, it becomes an 'n-type' semiconductor ('n' because the excess negatively charged electrons carry the current within the material). Alternatively we can dope the silicon with an element like boron which has one less electron. Again the boron substitutes for the silicon but to establish its covalent bonding pattern with the surrounding atoms, it needs to grab one electron. So it pulls one out of the surrounding crystal leaving behind a deficiency of electron. This 'missing electron' really behaves like a quasi-particle. It too can move through the crystal (it is really, of course, other electrons that are moving, but it works to think in terms of the 'hole' moving) and behaves as though it had a positive charge (a missing negative particle is a positive species). Once again the electrical conductivity of the semiconductor is enhanced but the material is now a 'p-type' semiconductor because of the excess of positively charged holes.
We can therefore 'tune' the electrical properties of silicon by adding these very low levels of impurities (typically less than one in a million silicon atoms will be replaced by the impurity). And fascinating phenomena follow when 'p' and 'n' materials are put together. thus a p/n junction has 'rectifying' action. It only allows electrical current to flow in one direction - a vital component in electrical circuitry. Indeed, the discovery of the rectifying action of p/n junctions by Bardeen and Shockley heralded the modern electronic age. The computing technology which has so transformed our lives in recent years, the computers that were used to generate the images here, all rely on the possibility of controlling the electrical properties of crystalline silicon by low levels of impurities.
 |
More complex but equally important types of disorder are present in 'line' defects known as 'dislocations', which involve a localised fault in the mode of packing of the crystal, for example the incomplete insertion of an extra layer of atoms. These species, which may be created when the crystal grows and which are introduced by heat or mechanical damage, drastically influence the mechanical behaviour of the material; they allow the material to flow and distort and that presence in high concentrations can lead to the failure of the material.
The science of defects in solids has progressed enormously over the last forty years. Indeed, the whole field of order and disorder in solids has now reached a sophisticated level of understanding. Interestingly, some solids tolerate very high levels of disorder while remaining crystalline, while for others only low concentrations of defects are present even at the melting point. But once again, we can understand these contrasts in terms of the balance between the energy required to create the disorder and the entropy that is gained on its creation.