Liquids and Glasses
 |
Disorder reigns at high temperatures. When the temperature rises, the price we have to pay for the entropy gain on achieving greater disorder becomes lower and lower; the entropy loss to the surroundings caused by the withdrawal of energy in creating the more disordered state drops with the rising temperature. Most solids therefore melt at higher temperatures (although some 'sublime', that is go straight to a gaseous state) to form liquids which are dense but disordered arrangements of atoms in three dimensions.
The densities of liquids are usually less than those of solids (although there are exceptions, as we will see later) - but only slightly so. Atoms and molecules are therefore roughly the same distances apart in liquids and solids. So how are they arranged? Does disorder reign supreme? The answer to the latter question is a definite no. Liquids do have structures. We know about structures of liquids from the scattering from them of X-rays and neutrons, although the interpretation of the results in terms of structure is more difficult than for crystalline solids, as discussed below. These experiments show that the atoms or molecules in liquids normally have well defined coordination numbers (i.e. they are surrounded by a well defined group of other atoms) and the coordination numbers are commonly similar to those in the corresponding crystalline solids. More distant coordination shells can often also be defined. What is lost in the liquid compared with the crystalline solid is the long range order. Liquid structures are not periodic: they cannot be defined in terms of a unit cell which is repeated regularly in three dimensions. But they do have short range order. So if, like "Maxwell's Demon", we could sit on an individual atom or molecule, as we looked out we would see close to us a reasonably well defined structure; but as our gaze went further into the liquid, we would lose increasingly any perception of order and structure. But when our demon moved to a crystalline solid, the perception of order would extend indefinitely.
The loss of long range order is shown dramatically by the changes on melting in the patterns created by the scattering of X-rays (or neutrons) from a substance. We recall that crystals act like diffraction gratings: X-rays are scattered at sharply defined angles. The disordered liquid does not behave like a grating: instead of sharp, well defined scattering angles, broad and diffuse peaks are observed, as shown above. But by analyzing the scattering, we can learn about structure, especially the "short range order", of the liquid.
 |
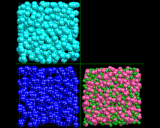
The computer is again a helpful tool in developing models for the complex structure of liquids. Of course in modeling liquids we must take into account the fact that they are dynamic assemblies: the atoms and molecules are in constant motion - a point which we will return to later. The molecular dynamics technique is therefore the ideal tool for exploring their properties, and indeed, this technique has contributed enormously to our understanding of the liquid state. Thus the images above show computer models - essentially snapshots - of the structures of three representative liquids: the first, liquid argon, comprises atoms interacting via weak van der Waals forces; the second, liquid nitrogen, contains distinct nitrogen N molecules; the third, molten salt (sodium chloride) contains ions. In each case, there is short range order with coordination numbers close to those in the corresponding solid; but the lack of long range order in the models is apparent.
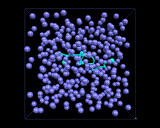 |
The computer models also allow us to explore the molecular motions which are a key feature of the liquid state. Atoms and molecules are, of course, in constant motion in crystals, these motions are essentially vibrations about well defined fixed sites. There are no fixed sites in liquids, and the atoms and molecules can diffuse through the liquid. They do so in a random manner as is shown by the computer generated trajectory illustrated in the snapshot above for the migration of an argon atom in liquid argon. Thus random, chaotic (or Brownian) motion is a vital feature of the physics of liquids, in contrast to crystalline solids, whose ordered structures lead to cooperative motions. Of course rates of diffusion depend on temperature, increasingly rapidly as the temperature rises and the atoms and molecules gain more energy. They also depend, as we might expect, on the shape of the molecule and the forces acting between the molecules. Diffusion will tend to be slower with long molecules which wrap around each other and between molecules which have very strong intermolecular forces. And as the molecular motion becomes slower, the liquid becomes more viscous. A simple but good example of the influence of molecular shape on the physical behavior of the corresponding liquid is provided by the paraffins - simple chains of carbon atoms bonded to hydrogen. As the chain gets longer, we change from gases, like methane (CH4) and ethane (C2H6) at ambient temperatures and pressures, to runny liquids (for the C6-C10 fraction - as in petrol/gasoline) to viscous liquids for C12-C20 to tars (which are so viscous that they begin to resemble solids) for longer molecules. Indeed, if the molecular motion becomes so slow that in effect the atoms and molecules have ceased to diffuse, we will have a material whose structure is that of a liquid, but many of whose physical properties are those of a solid; we will have made a glass, the topic of our next inquiry.
Amorphous Structures - Solids Without Periodic Patterns
Glass manufacture is one of the oldest technologies. The ancient worlds in both the East and West manufactured silicate glasses; and glass was a widely used material in the Mediterranean civilization dominated by the Romans for seven hundred years. Modern methods of making glass merely refine the procedures discovered in these earlier ages. A source of silicon dioxide (which may be sand which normally contains a high proportion of quartz) is mixed with a metallic oxide, such as soda (sodium carbonate) or lime (calcium oxide); these are sources of metal and oxygen ions which will react with the silicon dioxide to form silicates. The reacting mixture is heated and melted to form a molten silicate. The melt is then quenched - simply by pouring out of its crucible or, more drastically, by plunging into water. It cools rapidly and reverts to a solid. But it is a different type of solid from the crystalline precursors. For, as we have learned, on melting the atoms lose their ordered arrangements. And liquids are dense, disordered structures in which atoms are in constant motion. During the "quench" the atoms or molecules in the liquid have insufficient time for the intricate rearrangements and restructuring needed to make an ordered crystal; the solid freezes as the atoms slow down; and it sets into a disordered structure that is a frozen liquid. Long range order has been lost.
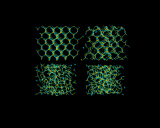 |
These strange structures have their own beauty. The images above illustrate computer generated models for glassy systems generated by Behnam Vessal. The first shows the melting of silicon dioxide. The following image shows models of silicate glasses (this image is larger if you follow its link); where we see that the metal ions are dispersed in homogeneously in the silicate matrix, which has profound consequences for the properties of the material.
 |
Highly Structured Liquids
All liquids, we have seen, have 'short range' structures apparent in the well defined coordination shells of the atoms, ions or molecules. In some liquids this ordering may become particularly pronounced, either because of specially strong forces acting between the molecules or because of the molecular shape.
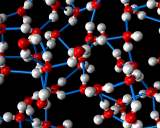
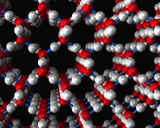
An example of the former is provided by the commonest liquid of all - at least in our environment - that is water. Water molecules interact ia strong hydrogen bonds in which the positively charged hydrogen atoms of one molecule stick to the negatively charges oxygen atoms of another. This hydrogen bonding promotes a tetrahedral coordination around the water molecules. Indeed, such structures are the basis of the complex crystal chemistry of ice, one of whose many structures is illustrated above. These structures persist into the liquid phase when ice melts. The structure of water just above the melting point of ice as revealed by molecular dynamics simulations is also illustrated.
 |
 |
 |
In the liquid, the hydrogen bonded networks which stabilize the very open structure of ice have partially collapsed, which is responsible for the remarkable increase in density of ice on melting. As the temperature increases, the hydrogen bonded network breaks up more and more but is still present right up to the boiling point of the liquid.
Other hydrogen bonded liquids also show pronounced structures, as in liquid ammonia (NH3) and liquid hydrogen fluoride (HF), a simulated structure of which are shown, where we see the tendency of hydrogen bonding to stabilize linear arrays of these molecules.
 |
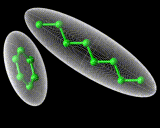
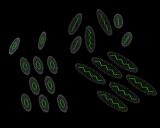
The second and even more remarkable class of structured liquids are liquid crystals. These are formed by molecules which are very 'non-spherical', that is either very long or very flat. Let us consider the long molecules first. If the molecules are relatively rigid as well as long, then they will tend not to wrap round each other but to line up, as we show schematically above. This alignment may spread over large distances involving hundreds or more molecules. it will promote order perpendicular in direction to the long axis of the rod-like molecules. However, there will be far less tendency to order in directions parallel to the axis of the rods. And in this direction the liquid will remain strongly disordered. So we have a system that tends to manifest order, but not in all dimensions - a liquid crystal.
 |
 |